Cochlear implants can help someone with serious hearing loss better understand human speech, converse on the phone, enjoy music and watch television. When successful, the device allows a user to perceive different types of sounds, such as doors slamming and dogs barking.
In the United States, however, it is estimated that only 10 percent of those who could benefit from the technology pursue implantation. Results with cochlear implants have been successful in general, but many cochlear implant recipients continue to have poor ability to understand human speech. Outcomes range from near normal ability to understand speech to no hearing benefit at all.
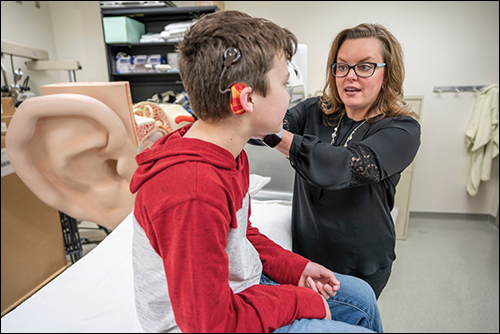
A team of Vanderbilt University and Vanderbilt University Medical Center researchers will reduce uncertainty with advanced patient-specific cochlear implant programming. The team received a five-year, $3.1 million National Institutes of Health grant in June 2020 to develop computational models for simulating how the cochlear implant activates the auditory nerves for individual patients. That follows a $3.9 million NIH grant in 2019 with many of the same researchers to develop customized implant programming for children with hearing loss.
Cochlear implants are small electronic devices with an external portion that sits behind the ear and a second portion surgically placed under the skin. The device uses an array of implanted electrodes to stimulate auditory nerves and induce hearing sensation.
The implants use from 12 to 22 electrodes, depending on the manufacturer. Although the implanted electrodes can be seen on a CT scan, the nerve cells they stimulate are not easily identified. Traditionally, all the electrodes are turned on and programmed to stimulate any surrounding nerve cells.
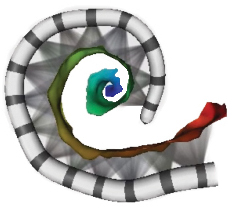
Sub-optimal nerve stimulation has been a big part of the variability in cochlear implant outcomes, but existing approaches for estimating how the electrodes stimulate the nerves on each patient have not been reliable enough to help audiologists make programming adjustments for consistent improvement. The vast majority of programming settings for commercial cochlear implants are left on the default values, with the ability to customize them untapped.
The new models will enable development of next-generation programming strategies to identify settings that greatly improve sound quality compared to the traditional programming approach.
Jack Noble, assistant professor of electrical engineering and computer science, leads the team and is the principal investigator. Co-investigators are Rene H. Gifford, professor of hearing and speech and director of the Division of Audiology’s Cochlear Implant Program; Robert Labadie, MD, professor of otolaryngology, and Cornelius Vanderbilt Professor Benoit Dawant, professor of electrical engineering. All are Vanderbilt Institute for Surgery and Engineering affiliates.

“Even among the most successful cases, restoration to normal auditory fidelity is rare,” Noble said. “It is estimated that less than 10 percent of those who could benefit from this technology pursue implantation, in large part due to the high-degree of uncertainty in outcomes.”
Outcomes also depend on successful positioning of the implant during surgery. The multidisciplinary team has developed image-guided techniques for more accurate detection of the location of implant electrodes relative to the auditory nerve cells they stimulate. The strategy supports deactivation of electrodes that imaging information suggests create overlapping stimulation patterns and audio “noise.”
The image-guided methods have significantly improved the quality of hearing for cochlear implant users. Now, Noble said, the five-year grant will enable “new, more advanced patient-custom programming strategies using novel methods for comprehensive patient-specific modeling of neural stimulation with cochlear implants.”