REGENERATIVE MEDICINE
Parsing T-cell activation is precursor to manipulating immune response to better fight diseases
For more than a decade Matt Lang and collaborators across the U.S. have worked to recreate key components of T-cells and how they know when to start fighting disease. Conventional wisdom suggested that T-cells formed regular, force-free bonds with infected cells, and in doing so caused the chain reaction of immune response. The team slowly built on its groundbreaking and controversial 2009 work that suggested the opposite—that the T-cell receptor required a force of some kind to activate.
In 2017 they finally measured the near immeasurable, down to a piconewton of force. They showed a directional nudge of ~10 piconewtons will launch the cascade of interactions in T-cell response. A piconewton is roughly the force exerted by dropping 1/1,000th of an eyelash, but it had been enough to cause a tsunami of dissent. Because they bucked decades of thinking, their findings were highly controversial and required a paradigm shift in research aimed at manipulating T-cell response.
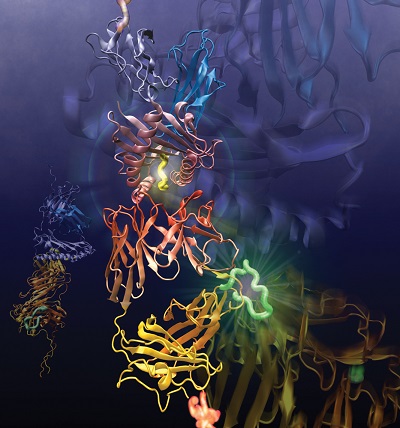
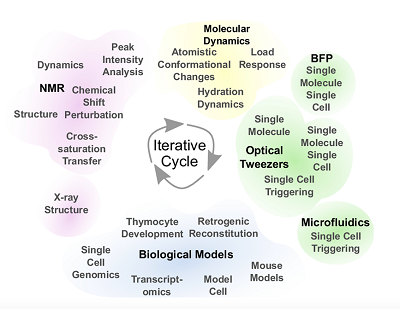
Their meticulous, painstaking work just received a big green light. The National Institute of Allergy and Infectious Diseases, the division of the National Institutes of Health that studies COVID-19 and seasonal flu, awarded the group a combined $11 million in the form of a rare and highly coveted program P01 grant. Managed by the Dana-Farber Cancer Institute, the five-year grant includes three synergistic projects and three cores. All of them involve characterizing the biological and structural features of T-cell signaling in even greater detail.
The first project uses a panel of T-cells and receptors sensitive to influenza virus to parse the conventional T-cell receptor response and understand the physical and chemical rules underpinning subcellular level triggering. The second follows T-cells as they are minted in the thymus and how they shuffle receptors through maturation to determine “the mechanical and physical rules that allow a cell to progress through developmental stages,” Lang said. The third project is about the receptor’s structure and dynamics where subtle changes among flavors of receptors will be mapped to their performance biologically such as repertoire, proliferation and protection. Lang has roles stitched within all three projects.
A more complete understanding of T-cell activation paves the way for manipulating this line of defense, switching them on to fight diseases or, for individuals with autoimmune disorders, blocking unwanted activation.
“We want to figure out not only what it takes to turn on but how that signal drives a cell into long-term memory,” said Lang, a professor of chemical and biomolecular engineering.
Lang was an associate professor at MIT in 2009 when the group first published together and suggested the T-cell receptor is a small machine that needs directional force to activate. The group, including experts in immunology, biophysics, molecular dynamics and structural biology, from Vanderbilt, Dana-Farber, Harvard and Texas A&M universities, kept at it.
“After we first published we would get attacked at conferences,” he said. “I was brand new to this field. We each were teaching each other our specialties and combined focus to bring people together over several different areas.
“It is about the synergy,” said Lang, who, with Ellis Reinherz of Dana-Farber, is a co-principal investigator on the program grant. “Little by little we started to build up evidence that there is a little machine in there.”
A T-cell is a type white blood cell, or lymphocyte, that develops in the thymus gland. This project focuses on CD-8 T-cells, which have ~30,000 copies of receptors unique to that cell on the cell surface. CD-8 T-cells play a big role in the adaptive immune response, an ancient feature that arose in jawed mammals 20 million years ago.
Although the grant targets seasonal flu (another ancient adaptive system) the work has application to cancer and other diseases including COVID. “If we figure this out, this will provide a way to trigger a cellular response and open new strategies for T-cell therapies and vaccine development,” Lang said.
They discovered and now seek to explain a set of rules in play that underlie the body’s immune response. “We are excited to do the work,” Lang said. “We are all in.”
