A research team that includes Vanderbilt engineers is the first to successfully separate two ions with minute small size differences, a major advancement in separation science with widespread potential application.
Their process is first to achieve solute-solute separation with sub-Angstrom precision. An Angstrom is one hundred-millionth of a centimeter, or one-tenth of a nanometer.
Use of nanofabrication for solute-solute separation makes the work significant as well. In most cases, nanofabrication is used to separate ions and small molecules from the solvent, not each other. Separating solvents from each other would allow easier recovery of valuable metals and other materials.
“Membranes capable of precise separation of ions and small molecules will have a transformative impact on the energy, water, chemical, and pharmaceutical industries,” the authors said.

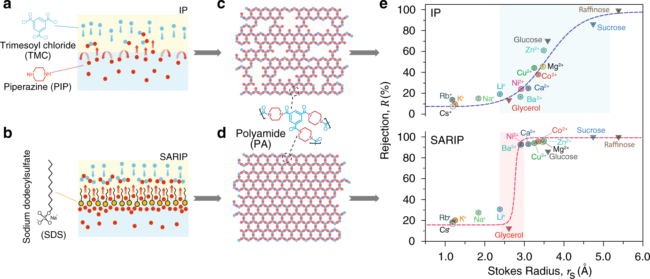
The key to achieving solute-solute separation, the authors discovered, is to use membranes with highly uniform pore size so they reject solutes larger than the pores but not just slightly smaller, said Shihong Lin, assistant professor of civil and environmental engineering and one of the project investigators. The work was reported in Nature Communications in April 2020.
“Such separations require membranes with highly uniform pore sizes to obtain precise molecular sieving and solute differentiation,” Lin said.
State-of-art commercial nanofiltration membranes are fabricated using interfacial polymerization, in which two chemical precursors, one in the water phase and the other in the oil phase, react. The reaction creates a thin film of polymer at the water/oil interface that acts as the active separation layer. This layer has Angstrom-scale pores, but the complex process happens within seconds and makes obtaining smaller, uniform pores very challenging.
The team’s novel method uses a dynamic, self-assembled network of surfactants to facilitate faster and more homogeneous diffusion of specific molecules, or monomers, across the water/oil interface, when the monomers bond with each other to form a polymer. The key to “surfactant assembly regulated interfacial polymerization,” or SARIP, as it is called, is in adding the right kinds of surfactants to promote the formation of a highly organized network of very narrow and highly uniform pore size at the water/oil interface.
The work results from an extensive international collaboration between Vanderbilt, the Suzhou Institute of Nano-Tech and Nano-Bionics of the Chinese Academy of Sciences, Yale University and several other institutions.