THE IDEA
Post-traumatic osteoarthritis—caused by degraded cartilage that cushions the ends of bones in joints—occurs after a joint injury. With the knowledge that PTOA will lead to earlier onset and faster progression of osteoarthritis following an injury, researchers including Craig Duvall, Cornelius Vanderbilt Professor of Engineering, set out to develop a drug for the prevention of PTOA initiation and progression.

The protein coding gene MMP13 is responsible for degrading cartilage, but researchers have yet to develop a therapy to inhibit it that doesn’t have adverse side effects. Duvall and his team of researchers, including former graduate student Sean Bedingfield and current graduate student Juan Colazo, were able to overcome this challenge by developing short interfering RNA-based drugs known as siRNAs.
“By using a siRNA-based approach, we are targeting the mRNA, the intermediate between genomic DNA and the functional protein that gets made,” said Duvall, also professor of biomedical engineering. “Because each gene sequence is unique, it is easier to selectively target and block translation of a specific protein using this class of drugs. And we chose to utilize this therapeutic strategy against MMP13 using a local injection directly into the injured joint.”
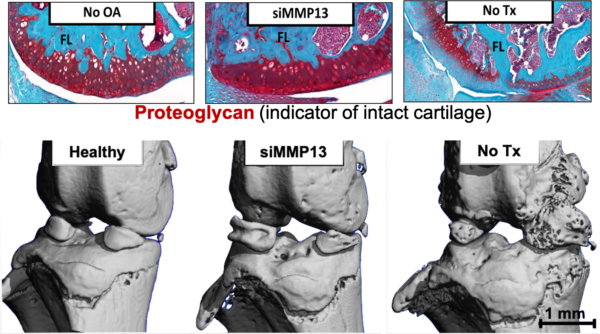
The team developed a nanoparticle loaded with the MMP13 siRNA that binds only to damaged cartilage affected by joint injuries. This targeted nanoparticle, when locally injected, stays in the joint longer to better combat early cartilage damage, Duvall said. This targeting approach also helps to further reduce potential undesirable effects elsewhere in the body.
Expanding on this work in a follow up article, the group used “packages” of nanoparticles to sustainably deliver the siRNA to the cells in the joint over time after treatment. With this technique, a single injection lasted for at least a month and reduced cartilage loss and bone spurs—known to be primary drivers of severe joint pain that ultimately causes patients to seek complete joint replacement.
WHY IT MATTERS
PTOA-causing injuries are most common among young athletes and military personnel, and osteoarthritis affects over 25 percent of those over 45 in the U.S. Current treatments like corticosteroid joint injections manage short term pain, but they may worsen cartilage loss when used as an ongoing therapy, Duvall said.
“Direct comparisons to treatment with the current clinical standard—steroids—showed that MMP13 silencing with the targeted nanoparticles had significant effects on reducing joint degeneration over steroid injections,” Duvall said. “This indicates that this approach has the potential to be developed as the first clinically available disease modifying osteoarthritis drug.”
WHAT’S NEXT
“We’d like to continue to explore bioadhesive nanoparticles that are simpler and more scalable to produce and that are longer lasting,” Duvall said. “We’d also like to show safety and efficacy in larger models as a stepping stone toward longer term application of MMP13 siRNA therapies in patients.”
FUNDING
This work was supported by the Department of Defense Peer Reviewed Orthopaedic Research Program, the National Institutes of Health, the Canadian Natural Sciences and Engineering Research Council, the Rheumatology Research Foundation, a VA Merit Award, the National Science Foundation Graduate Research Fellowship Program, the European Research Council under the European Union’s Seventh Framework Programme, and by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie Grant Agreement.
GO DEEPER
The article, “Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage” was published in the journal Nature Biomedical Engineering on August 19. Collaborators include Leslie Crofford, Professor of Pathology, Microbiology, and Immunology and Wilson Family Chair in Medicine at Vanderbilt University Medical Center and Karen Hasty of the University of Memphis and the University of Tennessee Health Sciences Center.
The follow up paper, “Top-Down Fabricated Microplates for Prolonged, Intra-articular Matrix Metalloproteinase 13 siRNA Nanocarrier Delivery to Reduce Post-traumatic Osteoarthritis” was published in the journal ACS Nano on August 19. Paolo Decuzzi, senior scientist and founding director of the laboratory of nanotechnology for precision medicine at the Italian Institute of Technology in Genova, was the primary collaborator on this work.