Researchers led by John T. Wilson, associate professor of chemical and biomolecular engineering and biomedical engineering, have developed a new approach using a molecularly designed nanobody platform that seeks to make immunotherapy more effective in the treatment of cancer.
The research, “Potentiating Cancer Immunotherapies with Modular Albumin-Hitchhiking Nanobody-STING Agonist Conjugates,” was published in Nature Biomedical Engineering on June 11, 2025.
 Immunotherapy is revolutionizing cancer treatment, but few patients benefit from the treatment, according to researchers. However, Wilson and his Immunoengineering Lab at Vanderbilt, along with collaborators at Vanderbilt University Medical Center, SOMBS, and the College of Arts and Sciences, aim to solve this problem.
Immunotherapy is revolutionizing cancer treatment, but few patients benefit from the treatment, according to researchers. However, Wilson and his Immunoengineering Lab at Vanderbilt, along with collaborators at Vanderbilt University Medical Center, SOMBS, and the College of Arts and Sciences, aim to solve this problem.
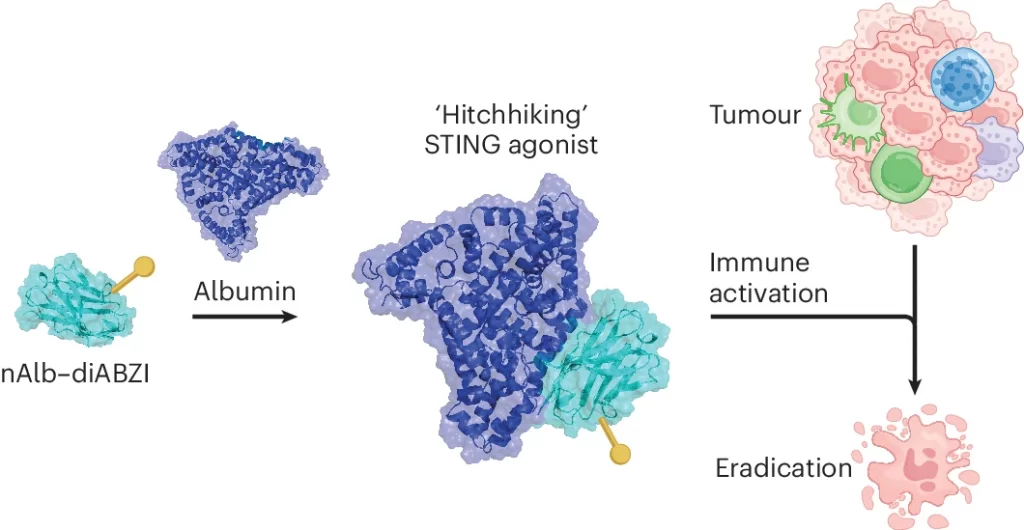
The team leveraged nanobodies – small antibody-like molecules that are derived from llamas and other cameloid species – that bind to serum albumin, the most abundant protein in the blood. Once injected, the nanobodies “hitchhike” on endogenous albumin molecules, a process that increases their circulation time but also takes advantage of albumin’s natural proclivity to accumulate at tumor sites.
The researchers then connected to this nanobody a molecule that activates the stimulator of interferon genes (STING) pathway. STING has been a promising target for improving cancer immunotherapy, but its effectiveness has been limited because it clears rapidly and doesn’t reach tumors well. But, by connecting the STING agonist to the albumin-binding nanobody, researchers were able to strongly improve its antitumor effects.
Another advantage of their technology is that it allows for multiple nanobodies to be strung together to introduce additional functionalities. To demonstrate this, the researchers added a second nanobody that binds to PD-L1, a protein expressed by cancer and immune cells that suppresses the killing ability of anti-tumor T cells. PD-L1 is the target of several clinically approved cancer immunotherapies. By linking the STING agonist to this bivalent nanobody – with one end binding to albumin and the other to PD-L1 – they found an increased delivery to tumor tissue and further improvements in effectiveness.
“We discovered this approach inhibited tumor growth in mouse models of breast cancer and melanoma, as well as improved response to currently approved immunotherapies such as immune checkpoint and inhibitors and adoptive T cell therapy,” said Wilson, the Ingram Associate Professor of Cancer Research who also co-leads the Host-Tumor Interactions Program of the Vanderbilt-Ingram Cancer Center.
Other research team members are Blaise R. Kimmel, Karan Arora, Neil C. Chada, Vijaya Bharti, Alexander J. Kwiatkowski, Jonah E. Finkelstein, Taylor L. Sheehy, Lucinda E. Pastora, Hayden M. Pagendarm, Payton T. Stone, Lauren A. Hubert, Ann Hanna, Emily N. Arner, Brandie C. Taylor, Katherine N. Gibson-Corley, Jeffrey C. Rathmell, W. Kimryn Rathmell, Justin M. Balko, Jinming Yang, Ebony Hargrove-Wiley, Barbara M. Fingleton, Ann Richmond, Jody C. May, and John A. McLean.
Contact: Lucas Johnson, lucas.l.johnson@vanderbilt.edu