A Vanderbilt engineering researcher has shown that combining an enhanced vibrational spectroscopy technique with tagged gold nanostructures can detect important tumor immunomarkers – a significant step toward predicting which patients would benefit from immunotherapy.
The study by Rizia Bardhan, assistant professor of chemical and biomolecular engineering, involved breast tumors but the work has broader relevance. Overexpression of one of the biomarkers, PD-L1, is also seen in melanoma, renal, colon, and non-small cell cancer, among others. The study also successfully and simultaneously diagnosed a second biomarker, epidermal growth factor receptor (EGFR), in triple-negative breast cancer, a highly aggressive disease.
Retrieving PD-L1 biomarker from preserved tumor tissue is very difficult and can result in misdiagnosis. Furthermore, traditional histology of biopsy samples typically provides only qualitative information.
The study, Bardhan said, “highlights the potential of surface-enhanced Raman spectroscopy (SERS) with dose-controlled gold nanostructures to transform diagnosis of cancer patients and enable patient-tailored therapies.”

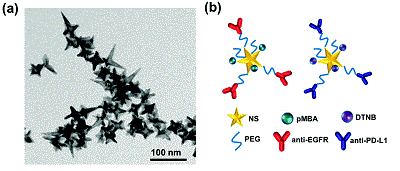
In this innovative work, led by graduate student Yu-Chuan Ou, Bardhan’s team demonstrated that both biomarkers can be detected with SERS in real time in vivo in living tumors tagged with gold nanostars. Further, the biomarker status was quantified ex vivo in whole tumor sections with SERS and the researchers achieved near-cellular level resolution with high accuracy.
“This would not replace traditional histology but complement it,” Bardhan said. “Incorporating this multiplexed diagnostic technique with histology can provide better prognosis, and important information for both treatment planning and patient monitoring.”
Cancer immunotherapies that inhibit checkpoint receptors such as PD-L1 are safer than chemotherapy and radiation and show better patient outcomes. But fewer than 25 percent of patients who receive immunotherapy to block PD-L1 currently respond to it. “This expensive antibody treatment also has toxic side effects, and the only way to measure whether patients are responding to treatment is repeated, invasive biopsies” Bardhan said.
Using this multimodal technique in tandem with classic analysis of tumor samples from biopsies spares patients from a treatment that won’t help them, she said.
The research is in its early stages and involved mouse models but it lays the foundation for work Bardhan is doing to combine two modes of analysis – SERS and PET scanning with these tagged gold nanostars.
Earlier this year she received a prestigious Congressionally Directed Medical Research Programs Career Development Award to develop such a multimodal multiplexed imaging platform for melanoma diagnosis and treatment evaluation. This award will fund research into a quicker, more accurate prediction tool to tailor these immunotherapies to melanoma patients who will respond to the treatment and determine an alternative plan for non-responders.
Gold nanoparticles are biocompatible and safe, and are already in phase III clinical trials to treat multiple cancers. They have recently shown complete remission in prostate cancer patients.
This work was done in collaboration with Orrin H. Ingram Professor of Engineering Anita Mahadevan-Jansen, professor of biomedical engineering. The article, “Diagnosis of immunomarkers in vio via multiplexed surface enhanced Raman spectroscopy with gold nanostars,” was recently published in Nanoscale, a journal of the Royal Society of Chemistry (United Kingdom).