Parker Hannifin Corporation received a gold award in the Rehabilitation and Assistive Device category for its Vanderbilt-designed Indego® exoskeleton at the 2016 Medical Design Excellence Awards ceremony June 14 at the Jacob K. Javits Convention Center in New York City.
The 2016 MDEA Juror Panel selected 38 exceptional finalists in nine medical technology product categories.
The exoskeleton was developed by Michael Goldfarb with a team of engineers and students in his Center for Intelligent Mechatronics. The technology portfolio is licensed by Parker Hannifin for commercialization. In March 2016 the U.S. Food and Drug Administration (FDA) gave clearance to market and sell the exoskeleton for both clinical and personal use in the United States.
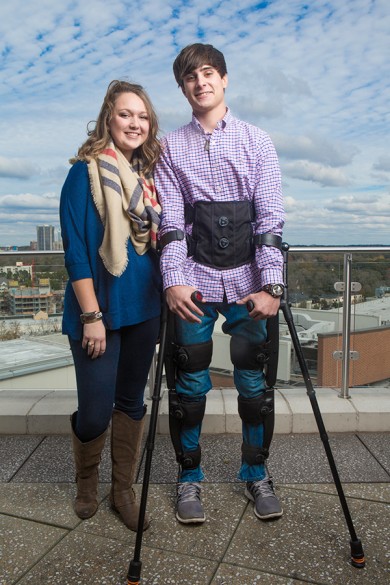
Indego is a powered lower limb exoskeleton enabling people with spinal cord injuries to walk and participate in over-ground gait training.
The device is the result of an intensive, 10-year effort. It acts like an external skeleton. It straps in tightly around the torso. Rigid supports are strapped to the legs and extend from the hip to the knee and from the knee to the foot. The hip and knee joints are driven by computer-controlled electric motors powered by advanced batteries. Patients use the exoskeleton with walkers or forearm crutches to maintain their balance.

“You can think of our exoskeleton as a Segway with legs. If the person wearing it leans forward, he moves forward. If he leans back and holds that position for a few seconds, he sits down. When he is sitting down, if he leans forward and holds that position for a few seconds, then he stands up,” Goldfarb said.
The MDEA is the medical technology industry’s premier design competition, and its panel of third-party jurors search worldwide for the highest caliber medical devices that save lives, improve patient health care and transform medical technology.
The MDEA Juror Panel selects products based on design and engineering innovation; function and user-related innovation; patient benefits; business benefits; and overall benefit to the health care system. The MDEA jury is comprised of a balance of practicing doctors, nurses, and technicians alongside industrial designers, engineers, manufacturers, and human factors experts.
Contact:
Brenda Ellis, (615) 343-6314
Brenda.Ellis@Vanderbilt.edu
Twitter @VUEngineering