A new study by Vanderbilt researchers demonstrates the ability to initiate chemical reactions by cooling materials instead of heating them— a counterintuitive process that could open new vistas for applications ranging from monitoring shipping conditions to developing smart clothing that guards against dangerously low temperatures.
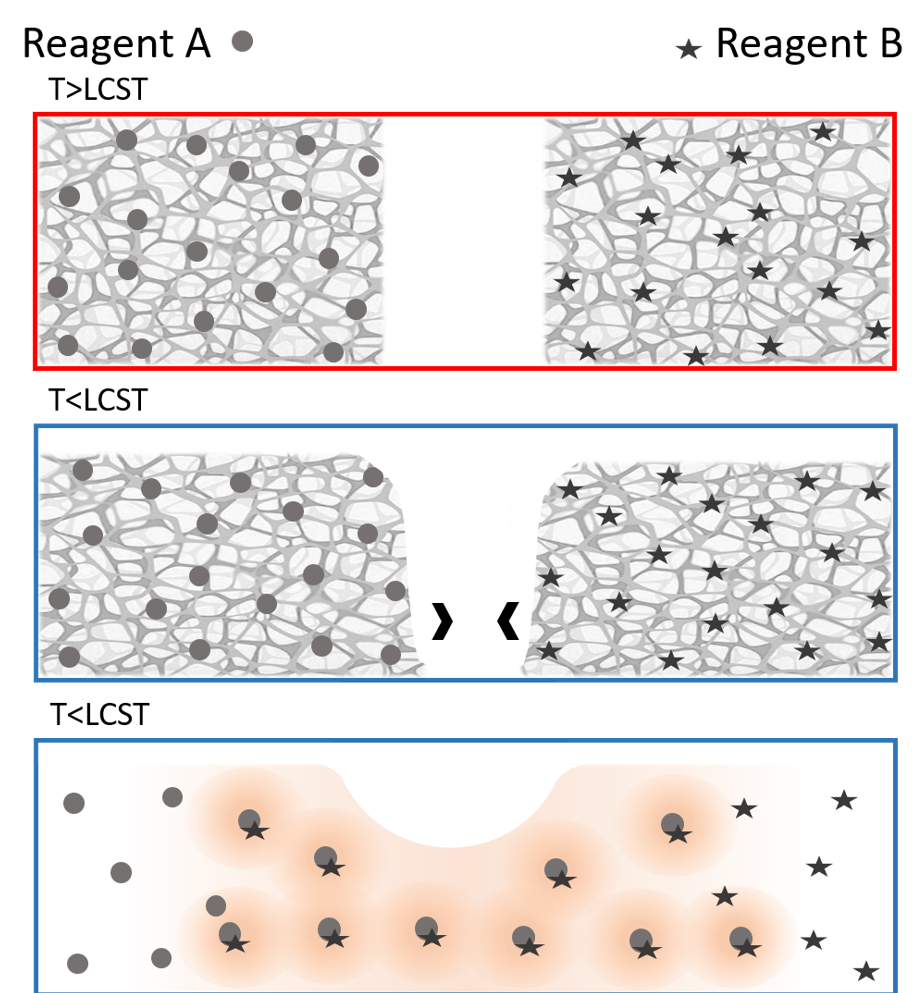 The paper, published in August by the journal RSC Advances, describes several experiments in which a material (methylcellulose) known for converting from a gel to a solution as temperatures drop was used to initiate mixing of two different agents embedded within. Once the material “melted” from a gel to a solution at lower temperatures, the embedded agents combined to start a chemical reaction.
The paper, published in August by the journal RSC Advances, describes several experiments in which a material (methylcellulose) known for converting from a gel to a solution as temperatures drop was used to initiate mixing of two different agents embedded within. Once the material “melted” from a gel to a solution at lower temperatures, the embedded agents combined to start a chemical reaction.
Vanderbilt doctoral student Romario Lobban led the research alongside Leon Bellan, associate professor of mechanical and biomedical engineering.
“Normally, when you think about initiating a chemical reaction, it involves applying heat. This is the reverse of that,” said Bellan, whose Lab for Advanced Materials focuses on creating materials with unique properties. “I like that there’s a counterintuitive aspect to this work; it’s somewhat unexpected.”

Bellan said he sees multiple possibilities for future developments that would benefit from a material that could kick start an action in response to cold temperatures.
Researchers used the cooling method to initiate both a pH-indicator and an enzymatic reaction. They were also able to fine tune activation temperatures by adding salt to the gel material.
This research was supported by the National Institute of General Medicinal Sciences of the National Institutes of Health under award number R01GM141604.