Berries really do pack extra punch – increasing the voltage of spinach-derived biohybrid solar cells developed by Vanderbilt researchers by up to a factor of 20.
The interdisciplinary team discovered that combining a natural dye from blackberries with photosynthetic proteins extracted from spinach leaves creates a device that can produce vastly more voltage than a solar cell made from spinach protein alone.
Biohybrid solar cells that incorporate natural materials can become a cost-effective source of electricity if their photovoltage potential is increased. At this point, the performance of these devices is nowhere near that of today’s silicon solar cells. Biohybrid technology is at an early stage, comparable to silicon solar cells of 30 to 40 years ago that were limited to powering electronic watches and calculators.
The team, led by Professor of Chemical and Biomolecular Engineering Kane Jennings and Professor of Chemistry David Cliffel, reported its discovery in the journal ACS Applied Energy Materials. Their article, “Photosystem I Multilayer Films for Photovoltage Enhancement in Natural Dye-Sensitized Solar Cells,” was published online Jan. 31.
Their layered structure of the blackberry-spinach device also is a significant departure from the approach other groups have taken.
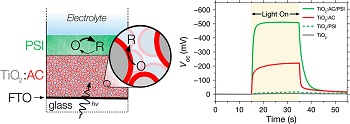
It consists of an electrode with a film of titanium oxide one micron (.00004 inches) thick, which is then soaked with a natural, anthocyanin dye extracted from blackberries. Anthocyanins are used to color textiles in shades of red, blue, purple and black. They are also approved in the European Union for coloring foods and beverages.
Next, the dye-soaked electrode is coated with 0.2 microns (about 20 layers) of photosystem I (PSI) molecules extracted from spinach leaves. PSI is the technical name for the protein found in plants responsible for the process of photosynthesis – capturing sunlight and transforming it into chemical energy that is the primary energy source for all living things on earth.
“We showed that this device produced about twice the photovoltage of the blackberry dye alone and 20 times that of the PSI alone,” Jennings said.

“We are discovering new ways in which PSI films can boost the performance of solar cells. If we can continue to improve the combined voltage and current that these biohybrid cells produce, then they could become a safe, healthy and relatively inexpensive source of power,” he said.
The 500 millivolts that the device generated is the highest photovoltage ever recorded by the group, which has been researching biohybrid solar cells for more than a decade.
One of the reasons the blackberry anthocyanins work so well with PSI proteins is because they absorb sunlight at complementary wavelengths. PSI absorption peaks at violet and red wavelengths while the dye absorption is strongest in green wavelengths. When they are combined, they can absorb a higher percentage of the incident light than they can individually.
“Understanding how to interface biological molecules and advanced electronic materials will be critically important in coming decades,” Cliffel said.
The team’s approach was informed by a mathematical model of multilayer PSI films developed by Maxwell Robinson as part of his doctoral thesis. (He is now a post-doctoral researcher in Chemical Engineering at the Massachusetts Institute of Technology.) The model was described in a paper published last December in The Journal of Physical Chemistry B.
Robinson’s model examined how PSI affects the local concentrations of small molecules called mediators. When light is shone on PSI, it separates positive and negative electrical charges with nearly perfect quantum efficiency. Mediators that diffuse through the cell to produce electrical current pick up these charges.

When positive and negative charge carriers meet, however, the charges recombine. The higher the recombination rate, the less power the cell produces. The model predicts that the PSI layer affects the concentration of mediator molecules in a way that reduces recombination rates, which helps explain the significant voltage increase in the layered design.
Two undergraduate students contributed to the research: Marie Armbruster, a Goldwater Scholar majoring in both chemical engineering and chemistry, and Avi Gargye, a chemical engineering major who began working on the project as a high school student.
The research was funded by U.S. Department of Agriculture grant 2013-67021-21029 and National Science Foundation grant DMR-1507505.
By contributor David Salisbury
Media Inquiries:
Pamela Coyle, (615) 343-5495
Pam.Coyle@Vanderbilt.edu
Twitter @VUEngineering